Oxygen in water
What is Dissolved Oxygen?
Dissolved oxygen refers to the level of free, non-compound oxygen present in water or other liquids. It is an important parameter in assessing water quality because it influenced the organisms living within a body of water. Ex: In limnology (the study of lakes), dissolved oxygen is an essential factor second only to water itself. A dissolved oxygen level that is too high or too low can harm aquatic life and affect water quality. Non-compound oxygen, or free oxygen (O2), is oxygen that is not bonded to any other element. Dissolved oxygen is the presence of these free O2.
molecules within the water. The bonded oxygen molecule in water (H2O) is in a compound and does not count toward dissolved oxygen levels. One can imagine that free oxygen molecules dissolve in water much the way salt or sugar does when it is stirred.
Dissolved Oxygen Units and Reporting
Dissolved oxygen is usually reported in milligrams per liter (mg/L) or as a percent of air saturation. However, some studies will report DO in parts per million (ppm) or in micromoles. (1 mg/L is equal to 1 ppm.) The relationship between mg/L and % air saturation varies with temperature, pressure, and, the salinity of the water. One micromole of oxygen is equal to 0.022391 milligrams, and this unit is commonly used in oceanic studies. Thus 100 micro-mole/L O2 is equal to 2.2 mg/L O2 Calculating Dissolved oxygen concentration from % Air Saturation To calculate dissolved oxygen concentrations from air saturation, it is necessary to know the temperature and salinity of the sample. Barometric pressure has already been accounted for as the partial pressure of oxygen contributes to the percent air saturation. Salinity and temperature can then be used in Henry’s Law to calculate the DO concentration would be at 100% air saturation. However, it is easier to use an oxygen solubility Chart. These charts show the dissolved oxygen concentration at 100% air saturation at varying temperatures and salinity. This value can then be multiplied by the measured percent air saturation to calculate the dissolved oxygen concentration.
Dissolved oxygen and aquatic life
Dissolved oxygen (DO) is one of the most important indicators of water quality. Oxygen dissolves in surface water due to the aerating action of winds, as a byproduct of aquatic plant photosynthesis. The concentration of oxygen less than 2 mg/L is called hypoxia and no oxygen levels refer to anoxia. Oxygen levels also may be reduced when there are too many bacteria or algae in the water. Oxygen enters water bodies primarily by transfer from the atmosphere across the air-water interface and to a lesser extent by the action of photosynthetic organisms. The rate of transfer of oxygen across the air-water interface is facilitated by increasing the surface area exposed to the atmosphere. The atmospheric transfer is the dominant mechanism for infusing oxygen into an aquatic system, the surface area to volume ratio is very important for establishing the baseline oxygen status for a given water body. Aquatic plants and algae also contribute dissolved oxygen to water bodies during daylight hours through photosynthesis. dissolved oxygen concentrations will typically be highest in the mid-to-late afternoon. When photosynthesis rates are increasing and will reach the lowest concentrations just before the sun rises the next morning due to respiration. Biological Oxygen Demand (BOD) is a measure of the potential for dissolved oxygen within a water body. Oxygen levels are reduced when there are too many bacteria or algae in the water. During the decay process of algae, the bacteria consume the oxygen dissolved in the water. This lead to a decrease in available oxygen in water bodies. Prolonged exposure to low dissolved oxygen levels may not directly kill an organism but may significantly increase its susceptibility to other environmental stresses and diseases. The lethal dissolved oxygen concentrations for fish are between 1 and 3 mg/L. Above 3 mg/L generally seem sufficient for many species. For the detection of the dissolved oxygen in water, there are several methods are used. They are an iodometric method and the membrane electrode method.
Dissolved Oxygen Saturation
In a stable body of water, dissolved oxygen is at equilibrium where water is holding as many dissolved gas molecules as it can in equilibrium. This condition is 100% air saturated. At equilibrium, according to Henry’s law, the percentage of dissolved gas is proportional to the partial pressure of that particular gas. The gas in the air dissolves into the water until equilibrium is reached. This process is hastened by aeration. Shallow water has 100% dissolved oxygen concentration whereas that of deeper water falls below 100% due to the less influence by aeration and consumption of oxygen by microbes in sediments or mud.
Factors affect the solubility of oxygen
Temperature: The solubility of oxygen rises slightly when the temperature decreases. When the temperature increases, the solubility reduces as the oxygen molecules which have greater kinetic energy, get eliminated from the water surface disrupting its intermolecular forces.
Pressure: As the atmospheric pressure increases, the solubility of oxygen gets increased because high pressure causes to trap more air in the water surface without escaping. Therefore water sources (sea water) at lower altitudes contain a high concentration of oxygen.
Salinity: When the salt concentration increases, the solubility of oxygen get reduces. Therefore, seawater has 20% less DO than fresh water that has a similar temperature and pressure.
How Can Water be More than 100% Saturated?
Aquatic respiration and decomposition lower DO concentrations, while rapid aeration and photosynthesis can contribute to supersaturation. During the process of photosynthesis, oxygen is produced and this can add to the dissolved oxygen concentration in the water, potentially bringing it above 100% saturation. Therefore, dissolved oxygen levels can easily be more than 100% air saturation during the day in photosynthetically active bodies of water. Supersaturation is also caused by rapid aeration which can often be seen beside hydro-power dams and large waterfalls. Rapid temperature changes could also make the water supersaturated.
Typical Dissolved oxygen levels
Dissolved oxygen refers to the level of free oxygen present in water. Water bodies receive oxygen from the atmosphere and from aquatic plants. Levels that are too high or too low can harm aquatic life and affect water quality. Dissolved oxygen concentrations are constantly affected by diffusion and aeration, photosynthesis, respiration, and decomposition. While water equilibrates toward 100% air saturation, dissolved oxygen levels will also fluctuate with temperature, salinity, and pressure changes. Water at lower temperatures should have higher mg/L of dissolved oxygen and higher dissolve while warmer, polluted waters will have lower mg/L. Healthy water should generally have dissolved oxygen concentrations above 6.5-8 mg/L and between about 80-120 %. Saltwater has a lower capacity (saturation level) to hold O2 than freshwater does. The lower the temperature and salinity level, the more oxygen the water can hold. Generally, the concentration of dissolved oxygen in ocean water is 7-8mg/L. Depending on species and stage of life, it is believed that a dissolved oxygen content of 5-6mg/L is sufficient for most marine organisms. While each organism has its own DO tolerance range, generally, DO levels below 3 mg/L are hypoxic, and DO levels below 1 mg/L are considered usually devoid of life. Fish growth and activity usually require 5-6 ppm of DO. Shrimp needs 5-7 mg/L DO to grow healthily and rapidly. Mussels need 0.7-0.8 mg/L and they suffer heavy mortality rates if DO concentrations dipped below this point. Generally dissolved oxygen levels below 3 ppm are stressful to most aquatic organisms. The low level of dissolved oxygen in water is a sign of contamination and is an important factor in determining water quality, pollution control, and treatment process. Just as low dissolved oxygen can cause problems, so too can high concentrations. Supersaturated water can cause gas bubble disease in fish and invertebrates. Significant death rates occur when dissolved oxygen remains above 115%-120% air saturation for some time.
Consequences of Unusual Dissolved Oxygen Levels
When the dissolved oxygen concentrations drop below a certain level, fish mortality rates will rise. Sensitive freshwater fish like salmon can’t even reproduce at levels below 6 mg/L. In the ocean, coastal fish begin to avoid areas where DO is below 3.7 mg/L, with specific species abandoning an area completely when levels fall below 3.5 mg/L. Below 2.0 mg/L, invertebrates also leave and below 1 mg/L even benthic organisms show reduced growth and survival rates.
A fish kill/ winter kill
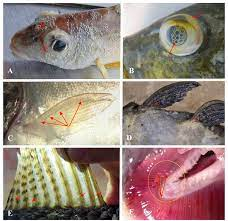
Gas Bubble Disease
figure 03: gas bubble disease
is caused by high oxygen concentrations. Supersaturated water can cause gas bubble disease in fish and invertebrates. Significant death rates occur when dissolved oxygen remains above 115%-120% air saturation for some time
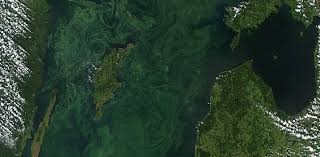
A Dead Zone
figure04: Algal blooms in the baltic sea
an area of water with little to no dissolved oxygen present. They are so named because aquatic organisms cannot survive there. These zones are usually a result of a fertilizer-fueled algae and phytoplankton growth boom. When the algae and phytoplankton die, the microbes on the seafloor use A stratification the oxygen decomposing the organic matter. These anoxic conditions are usually stratified, occurring only in the lower layers of the water. While some fish and other organisms can escape, shellfish, young fish and eggs usually die.
Dissolved Oxygen and Water Column Stratification
A stratification is an act of sorting data, people, and objects into distinct groups or layers and it can be studied in lakes, oceans, and estuaries. Considering lake stratification; the uppermost layer; epilimnion is warmer than other layers and the depth is dependent on the temperature exchange. In this layer generally algae and phytoplankton engage in photosynthesis and the dissolved oxygen in this layer is nearly 100% saturation. The next level is metalimnion, a transitional layer that fluctuates in thickness and temperature. If the light can penetrate this layer photosynthesis may occur and the dissolved oxygen layer increases. The next layer is hypolimnion. In this layer bacteria and fungi use dissolved oxygen to decompose organic materials which come from sunken dead algae and other organisms. The problem here is that the dissolved oxygen used in decomposition is not replaced because this layer does not contact with the atmosphere for aeration and photosynthesis. The next stratification is oceanic stratification which can occur both horizontally and vertically. In sub littoral zone dissolved oxygen levels may not fluctuate as much as in the littoral layer. In both these zones, many coral reefs occur and the dissolved oxygen concentration is nearly 100% air saturation. Beyond the damsel, the zone is bathyal, abyssal, and hadal plains, which are in low dissolved oxygen concentration. In vertical strata; the epipelagic zone is known as a photic zone where light penetrates. This is the layer with the highest level of dissolved oxygen due to wave action and photosynthesis. The last stratification is estuary stratification, which is based on salinity distribution because saltwater has less dissolved oxygen concentration than freshwater. The higher river flow increases the dissolved oxygen concentration in the water.






No comments:
Post a Comment